Overview:
Our Medical Grade Polishing Overmolding Molding Tooling Mold with Multi-Cavity Solutions represents the pinnacle of precision mold manufacturing for critical healthcare applications. Engineered specifically for producing Class II and III medical devices requiring flawless surface finishes and complex multi-material integration, these molds enable the production of components such as drug delivery seals, surgical instrument grips, and implantable device housings. Crafted from premium corrosion-resistant tool steels and utilizing state-of-the-art manufacturing technologies, each mold delivers exceptional dimensional stability, extended service life, and consistent performance through millions of cycles in validated cleanroom production environments.


Basic Information:
| Specification | Details |
|---|---|
| Material Options | S136, H13, NAK80, 420 Stainless Steel, Stavax ESR |
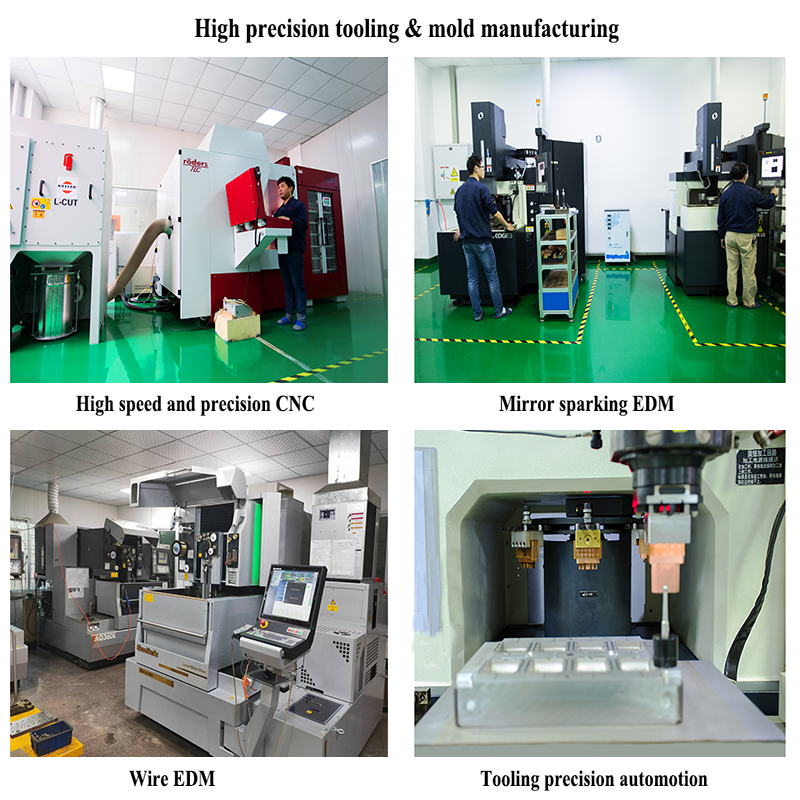
| Core Processes | 5-Axis CNC Machining, Mirror EDM, Precision Grinding, Slow Wire Cutting |
| Surface Finish | Medical-Grade Mirror Polish (Ra ≤ 0.025 μm) |
| Mold Type | Multi-Cavity Overmolding, Hot Runner, Stack Molds |
| Lead Time | Prototype: 15-25 Days / Production Mold: 5-8 Weeks |
| Tolerance | ±0.003 mm |
| Compatible Materials | Medical LSR, PEEK, PC, PP, PEI (Ulitem) |
| Cavity Configuration | 2-32 Cavities (Optimized for Balanced Filling) |
| Certification | ISO 13485:2016, ISO 9001:2015, FDA CFR 820 |
| Primary Applications | Surgical Tools, Implantable Housings, Drug Delivery Components |
Key Advantages:
-
Ultra-Precision Medical Compliance: Manufactured under ISO 13485 quality systems with full material traceability and validation documentation packages
-
Advanced Overmolding Capability: Precision-engineered gating and venting systems ensure perfect bonding between LSR and engineering thermoplastics
-
Optical-Grade Surface Technology: Proprietary polishing techniques achieve sub-micron surface finishes that prevent bacterial adhesion and facilitate sterilization
-
High-Yield Production Architecture: Scientifically balanced multi-cavity layouts with integrated hot runner systems ensure ±0.1% part weight consistency
-
Extended Operational Lifespan: Premium tool steels with specialized surface treatments withstand repeated autoclave sterilization and abrasive medical compounds
-
Comprehensive Technical Partnership: DFM analysis, mold-flow simulation, validation sampling, and production support throughout product lifecycle
Surface Treatment Options:
-
Medical-Grade Mirror Polishing (SPI A1): Achieves optical clarity for fluid visibility and easy de-molding
-
Plasma Nitriding & PVD Coatings: Enhanced surface hardness (HRC 65+) and chemical resistance against medical-grade polymers
-
Nanotech Surface Enhancement: Proprietary treatment reducing surface energy to prevent material adhesion in micro-features
-
Electropolishing & Passivation: Superior corrosion protection for molds used with saline-based compounds and sterilization
Quality Assurance:
Every medical mold undergoes exhaustive verification through our comprehensive quality management system. We employ ZEISS ACCURA 3D CMMs with 0.1μm accuracy for dimensional validation, white light interferometers for surface roughness verification, and industrial endoscopes for micro-cavity inspection. Mold-flow analysis and thermal imaging ensure perfect temperature distribution across all cavities. Each tool completes rigorous sampling runs with certified medical-grade materials, producing components that undergo full dimensional, functional, and cleanliness validation per ISO 14644-1 Class 7 cleanroom standards.
Our Factory:
Our dedicated medical mold manufacturing facility operates 28 advanced CNC machining centers (5-axis simultaneous), 12 mirror EDM systems with 0.5μm repeatability, and automated robotic polishing cells within climate-controlled cleanrooms. The integration of AGV material handling systems, real-time tool monitoring, and paperless manufacturing execution systems (MES) ensures complete process control and traceability. Our specialized medical mold division features separate temperature/humidity-controlled polishing rooms, ultrasonic cleaning stations, and Class 1000 assembly areas to maintain the stringent cleanliness standards required for implantable medical devices.